Chinese-made Omicron vaccine trials get going

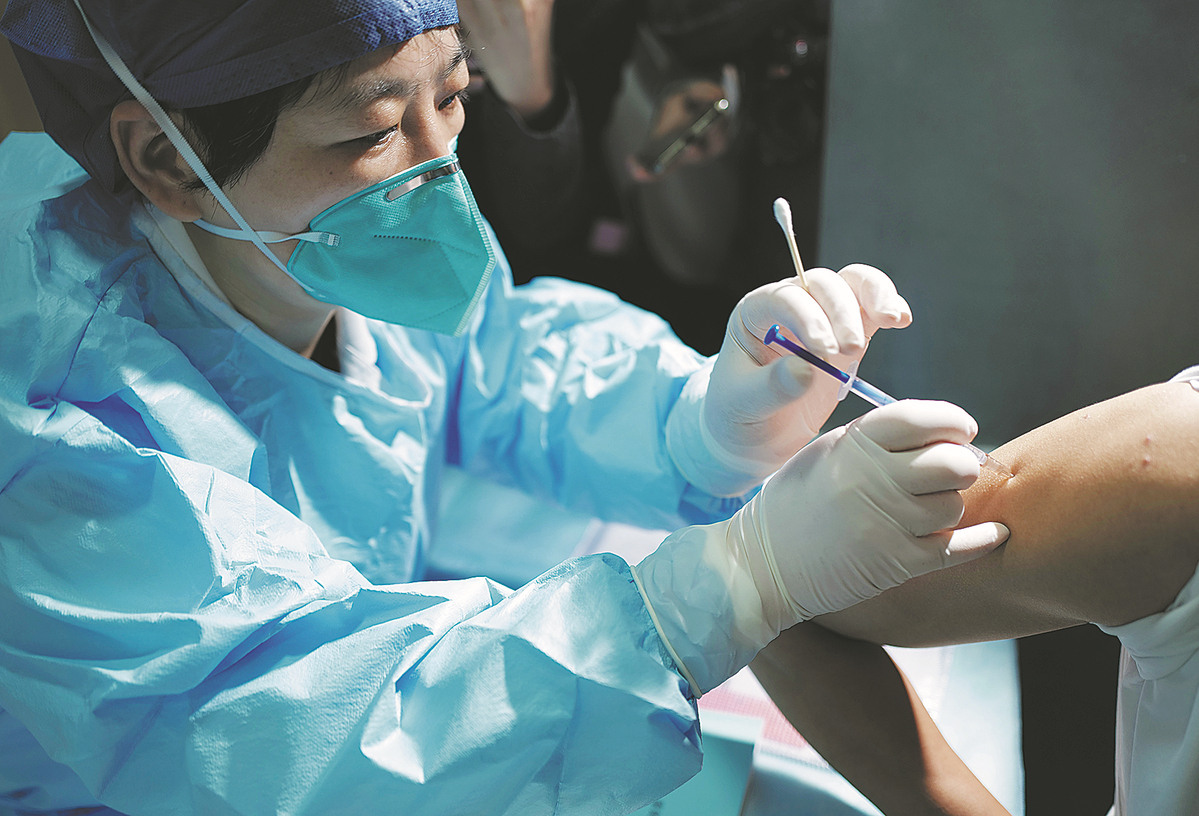
China began clinical trials for a vaccine designed specifically against the Omicron variant in Hangzhou, Zhejiang province, on Sunday, making it the first vaccine of its kind to enter human trials in the world against the highly transmissible COVID-19 strain.
China National Biotech Group, a subsidiary of Sinopharm, has been developing the vaccine since December 2021, the company said in a news release on Monday.
The National Medical Products Administration approved the clinical trials for the vaccine on April 26. Three days later, the United Arab Emirates also gave the greenlight for clinical trials.
The trial in China would consist of a randomized, double-blind study on people age 18 and older who have been inoculated with two or three doses of COVID-19 vaccines.
Yang Xiaoming, chairman of China National Biotech Group, said in the launch ceremony of the vaccine's clinical trial the Omicron variant is highly transmissible and elusive. He hopes the new vaccine will achieve success and benefit people around the world.
Zhang Yuntao, the chief scientist at the company, said the Omicron vaccine entering clinical trial represents a major development in the fight against COVID-19 in China. The trial will examine the safety and potency of the vaccine, he added.
- China issues orange alert for Typhoon Matmo
- Xi, Bangladeshi president exchange congratulations on 50th anniversary of ties
- Vibrant China during holiday: Traditional culture meets tech
- China activates emergency response as Typhoon Matmo approaches
- Over 230 anticancer drugs feature in national medical insurance catalog
- South China provinces activate Level-IV emergency typhoon response