Chinese research team launches clinical trial for invasive brain-computer interface

SHANGHAI -- A Chinese man who lost all four limbs in a high-voltage electrical accident 13 years ago can now play chess and racing games using only his mind, after a revolutionary procedure in Shanghai in which a brain-computer interface (BCI) device was implanted in his brain.
This is not a science-fiction premise, but China's first-in-human clinical trial of an invasive BCI, and an important step in the transition of this technology from laboratory research to clinical application.
The trial is a collaboration between research teams from the Center for Excellence in Brain Science and Intelligence Technology (CEBSIT) at the Chinese Academy of Sciences (CAS), and Huashan Hospital affiliated with Fudan University. Their work makes China the second country, after the United States, to advance invasive BCI technology to the clinical trial stage.
Since the device was implanted in March 2025, it has operated stably in the patient's brain, with no infection or electrode failure reported to date, according to the research team.
The team hopes the system could enter the market after receiving regulatory approval in 2028, with the potential to enhance the quality of life of millions of patients suffering from complete spinal cord injuries, double upper limb amputations, and amyotrophic lateral sclerosis.
BCI technology establishes a direct communication and control connection between the brain and the external world. It is not only a window to understanding the brain's information processing mechanisms but also a direction and means for treating diseases and exploring the next generation of human-computer interaction modes, said Shi Yongyong, deputy director of the CEBSIT.
Reading the information of electrical signals from the brain and using it to control external devices is a field of research with a history of two to three decades. However, in the past, bulky instruments were used, said Pu Muming, an academician of the CAS.
The process of miniaturizing and systematizing the large device is extremely challenging, Pu said.
The ultra-flexible neural electrodes developed by the team are incredibly fine, measuring only about 1 percent of the diameter of a human hair, according to Zhao Zhengtuo, head of the research team.
"This allows brain cells to barely 'perceive' their presence, minimizing brain tissue damage," Zhao said.
These electrodes enable high-density, large-scale, high-throughput, and long-term stable in vivo neural signal acquisition. They've been tested in rodents, non-human primates, and then the human brain, Zhao said.
The invasive BCI system developed by the team can stably acquire clear single-neuron signals. The implant measures 26 mm in diameter and less than 6 mm in thickness, roughly the size of a coin, making it half the size of the product developed by Elon Musk's Neuralink, according to Zhao.
Real-time decoding is a key of the BCI technology. This system completes the entire process of neural signal extraction, movement intent interpretation, and control command generation within tens of milliseconds, faster than the blink of an eye, said Li Xue, another leading researcher of the team.
Prior to human trials, the system's safety and functionality were validated in macaque monkeys. Following implantation surgery, the system operated stably with no infection or electrode failure observed. The implant was also safely replaced in the monkey experiment, proving feasibility of upgrading the device, Li added.
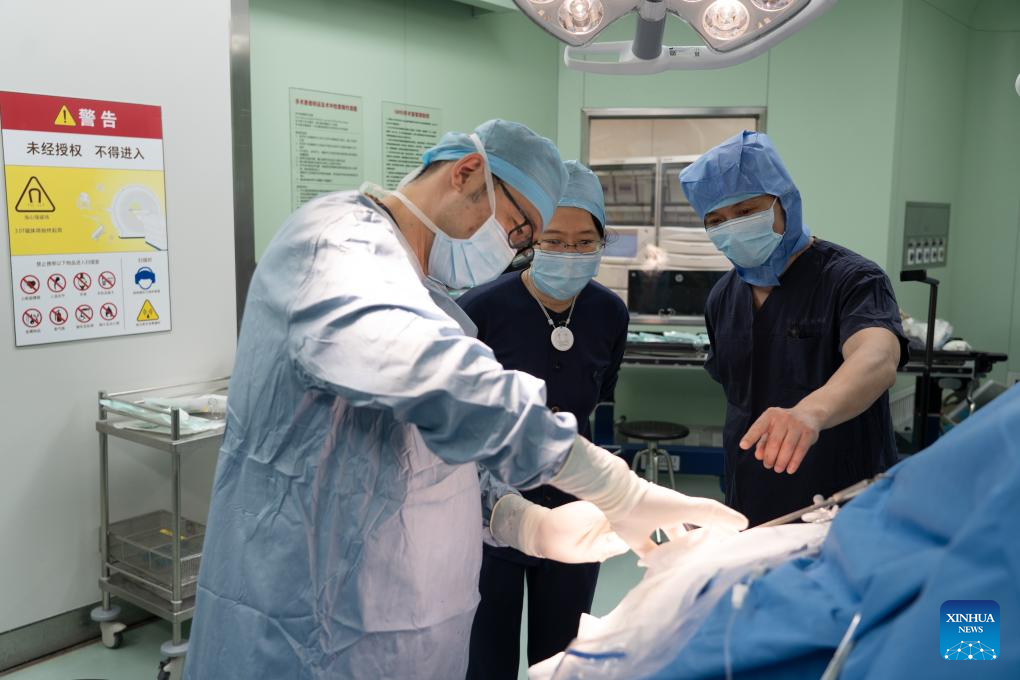
The implantation surgery utilizes minimally invasive neurosurgical techniques, effectively reducing risks and shortening recovery time. The operational procedure facilitates broader application, said Lu Junfeng, principal investigator of the team from the Huashan Hospital.
Accurate electrode placement is vital. Using multiple positioning methods and high-precision navigation, Lu's team implanted the ultra-flexible electrodes into the designated area of the patient's motor cortex.
The entire procedure was accurate to the millimeter, maximizing safety and efficacy, Lu said.
Currently, there are mainly three BCI technological routes: non-invasive, semi-invasive, and invasive. Non-invasive methods are entirely non-surgical, while semi-invasive and invasive approaches involve surgical procedures.
To explain the difference of these technical approaches, Lu gave an analogy: "It is like listening to a live broadcast of a soccer game. Non-invasive devices are like microphones placed outside the stadium -- people can judge the game's progress by the cheers but cannot accurately know what is happening on the field. Semi-invasive devices are like microphones hung above inside the stadium, allowing people to understand the game more clearly. Invasive devices are like microphones attached to the players and spectators, enabling audience to hear very clearly what the players and spectators are saying and to understand more accurately what is happening on the field."
Next, the team aims to enable the patient to control a robotic arm, allowing him to grasp and hold objects like cups. They will also explore controlling complex devices such as robot dogs and embodied intelligent robots to expand the life boundaries of the patient.
- Researchers launch clinical trial for invasive brain-computer interface
- Kazakh youths embrace e-commerce at China-Kazakhstan border
- China steps up preparations for Typhoon Wutip
- Exhibition highlights Yangtze River's influence on Chinese civilization
- China launches nationwide crackdown on misconduct in medical sector
- Blood donation rate rises, shortages remain